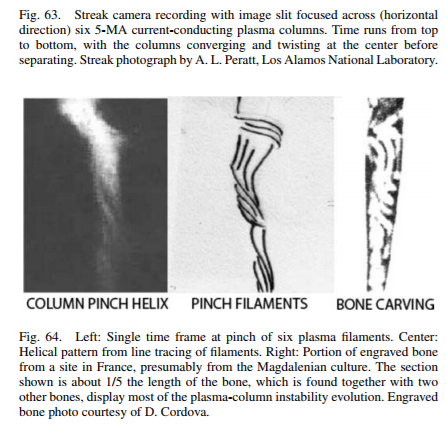
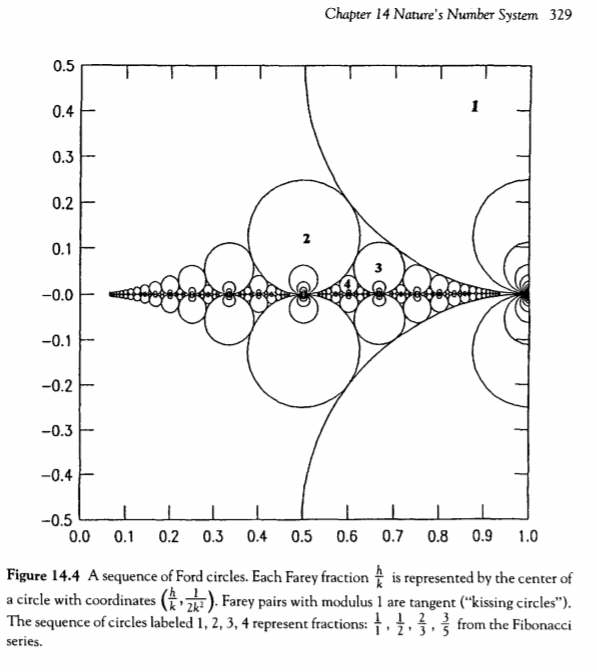
EU Meetup January 30, 2018
| Do electrons and protons maintain their identity ? 1 message |
| Juan Calsiano <juancalsiano@gmail.com> | Mon, Jan 29, 2018 at 1:39 PM | |
|
To: Edo Kael <edwinkaal00@gmail.com>
Cc: Jim Weninger <jwen1@yahoo.com>, "dj@argos.vu" <dj@argos.vu>
|
||
|
||
Scientists unveil new form of matter: time crystals
|
|||||||||
|
7:09 PM  |
  |
||
|
||||
Sent from my iPad
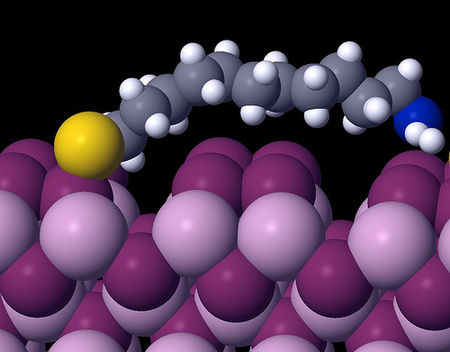
Sterically Stabilized Dispersion
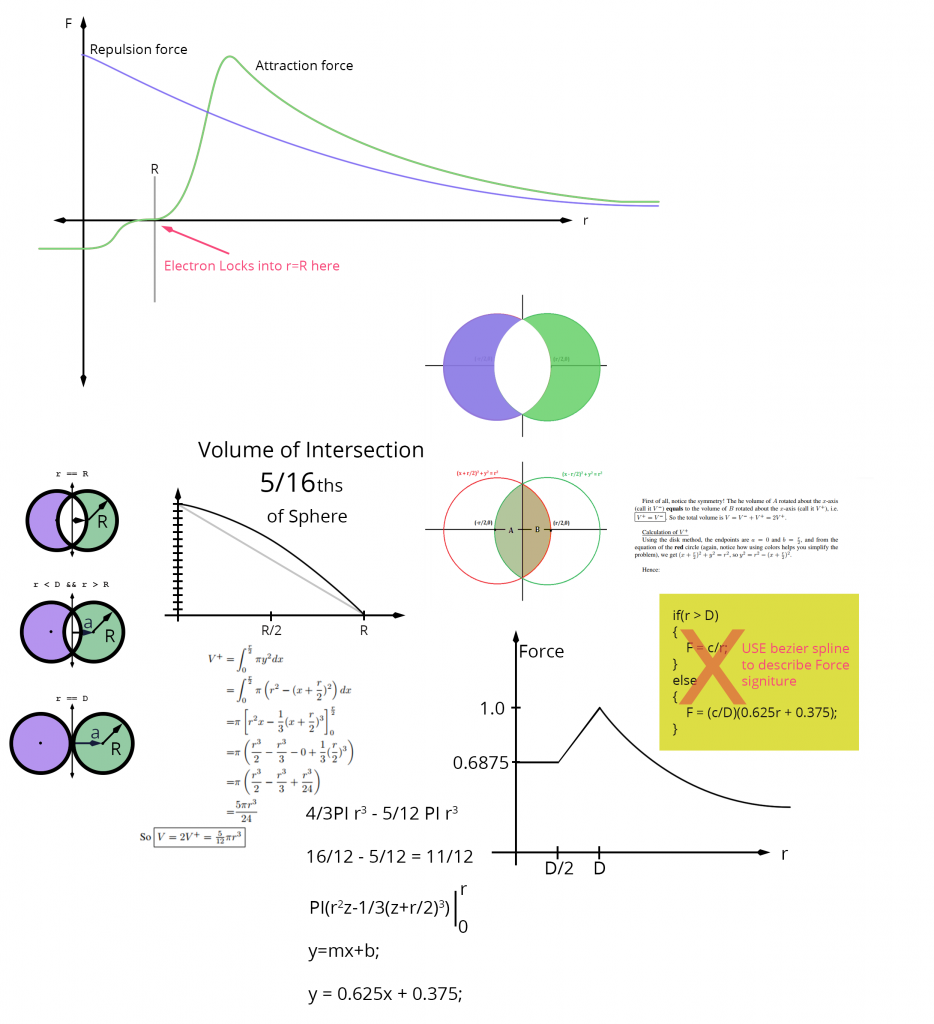
For sterically stabilized dispersions, the resulting energy-distance curve often shows a shallow minimum, Vmin, at a particle-particle separation distance h comparable to twice the adsorbed layer thickness delta. For a given material, the depth of this minimum depends on the particle size R and adsorbed layer thickness delta.
Hence Vmin decreases with increase in <math>\frac{\delta }{R}</math>, as illustrated in the figure above. This is because as we increase the layer thickness, the van der Waals attraction weakness so the superposition of attraction and repulsion will have a smaller minimum. For very small steric layers, <math>V_{\min }</math> may become deep enough to cause weak flocculation, resulting in a weak attractive gel.
On the other hand, if the layer thickness is too large, the viscosity is also increased due to repulsion. This is due to the much higher effective volume fraction <math>\Phi _{eff}</math> of the dispersion compared with the core volume fraction. We can calculate the effective volume fraction of particles plus dispersant layer by geometry and we see that it depends on the thickness of that adsorbed layer, as illustrated in the figure below.
The effective volume fraction increases with relative increase in the dispersant layer thickness. Even at 10% volume fraction we can soon reach maximum packing (<math>\Phi =0.67</math>) with an adsorbed layer comparable to the particle radius. In this case, overlap of the steric layers will result in significant viscosity increases. Such considerations help to explain why the solids loading can be severely limited, especially with small particles. In practice, solids loading curves can be used to characterize the system and will take the form of those illustrated below:
A higher solids loading might be achieved with thinner adsorbed layers but may also lead to interparticle attraction, resulting in particle aggregation. Clearly a compromise is needed: choosing an appropriate steric stabilizer for the particle size of the pigment.
Super Blue Blood Moon 2018:
During the early hours of Jan. 31, there will be a full moon, a total lunar eclipse, a blue moon and a supermoon. None of these things is all that unusual. What is rare is that they’re happening all together on one day.