To any doctor trained in today’s medical schools, the idea that the heart may not be a pump would, at first sight, appear to be about as logical as suggesting that the sun rises in the West or that water flows uphill. So strongly is the pump concept ingrained in the collective psyche that even trying to think otherwise is more than most people can manage. Yet Rudolf Steiner, a man not given to unscientific or slipshod thinking, was quite clear on the matter and reiterated time and again that the heart is not a pump. “The blood drives the heart, not the heart the blood.”
Ralph Marinelli* and his co-workers published a paper refuting the generally-accepted pressure-propulsion premise. For a start, they draw attention to the sheer volume of work which the heart would have to do if it were solely responsible for pumping inert blood through the vessels of the circulatory system. Blood is five times as viscous as water. According to the propulsion premise the heart would have to pump 8000 liters of blood a day in a body at rest and considerably more during activity, through millions of capillaries the diameters of which are sometimes smaller than the red blood cells themselves – a huge task for a relatively small, muscular organ weighing only 300 grams.
Once the questions start being asked, the anomalies in currently accepted dogma become apparent. For instance, if blood were pumped under pressure out of the left ventricle into the aorta during systole, the pressure pulse would cause the aortic arch to try and straighten out, as happens in any Bourdon tube pressure gauge. In practice the exact opposite happens; the curve increases, indicating that the aorta is undergoing a negative, rather than a positive, pressure.
Another paradoxical finding concerns the mechanics of fluid flow under pulsatile pressure. When a pressure pulse is applied to a viscous fluid in a closed vessel, the liquid initially resists movement through its own inertia. The pressure, therefore, peaks before the fluid velocity peaks. In the aorta, exactly the opposite happens where a peak flow markedly precedes peak pressure, a fact which was observed in 1860 by Chaveau and Lortet. So just what is going on inside the circulation?
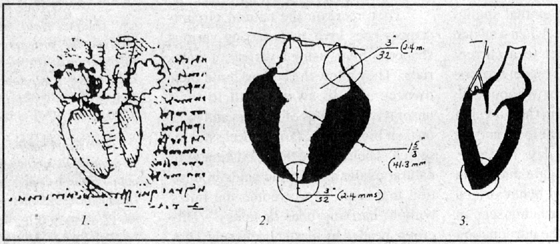 Misleading sketch of the heart by Leonardo do Vinci (1). The left ventricle wall is shown uniform in thickness as it would he in a pressure chamber. Actually the left ventricle wall thickness varies by about 1800% as Marinelli and his group measured in bovine hearts (2). The apex wall is so soft and weak that it can be pierced with the index finger. The peculiar variability in the ventricular wall thickness is not in keeping with the heart as pressure generator. However, Leonardo’s Notebooks has been used in most biology, physiology, and medical texts during the last few hundred years as well as in most modern anatomy texts in the last decades (3). Thus, false sketches have served to bear witness to a false premise.
Misleading sketch of the heart by Leonardo do Vinci (1). The left ventricle wall is shown uniform in thickness as it would he in a pressure chamber. Actually the left ventricle wall thickness varies by about 1800% as Marinelli and his group measured in bovine hearts (2). The apex wall is so soft and weak that it can be pierced with the index finger. The peculiar variability in the ventricular wall thickness is not in keeping with the heart as pressure generator. However, Leonardo’s Notebooks has been used in most biology, physiology, and medical texts during the last few hundred years as well as in most modern anatomy texts in the last decades (3). Thus, false sketches have served to bear witness to a false premise.
As Marinelli et al point out, the pressure-propulsion model of blood circulation rests on four major premises: (1) blood is naturally inert and must, therefore, be forced to circulate; (2) there is a random mix of formed particles in the blood; (3) blood cells are under pressure at all times; (4) blood is amorphous and is forced to fill its vessels and take on their form.
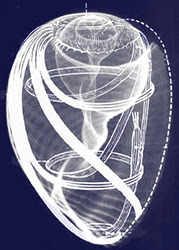
All of these premises can be shown to be faulty. For example, far from having a random mix of the blood components in vessels, the cellular elements arrange themselves in a highly organized flow pattern in which the heavier red blood cells flow nearest to the axis of the vessels while the lighter platelets are nearer to the periphery. All of the formed elements are surrounded by a sleeve of plasma which is in contact with the vessel wall. However, a major misconception about how blood circulates is the assumption that it flows in a laminar fashion, whereas in reality the main pattern appears to be a vortex. This leads to a whole new concept of circulatory dynamics–one which goes a long way towards explaining the close interaction between the heart and the blood– both of which are derived from the same embryonic material.
Clues to circulatory physiology are found in embryology. Two of the main embryological observations have been that the blood starts circulating before the heart has been fully formed and that it circulates in a spiraling fashion, as in the single-stage tube heart of the chick before the valves have developed.
Why are we concerned about the way in which the blood circulates and the heart as a pump paradox? Do we not already know enough about circulation in conventional terms for all practical purposes? No. Is all this really relevant? Yes. Not only should truth be sought for its own sake, but therapy based upon faulty premises can only be bad therapy.
A REFUTATION OF THE PRESSURE PROPULSION PREMISE OF HEART FUNCTION
Ralph Marinelli 1; Branko Fuerst 2; Hoyte van der Zee 3; Andrew McGinn 4; William Marinelli 5
1. Rudolf Steiner Research Center, Royal Oak, MI
2. Dept. of Anesthesiology, Albany Medical College, Albany, NY
3. Dept. of Anesthesiology and Physiology, Albany Medical College, NY
4. Cardiovascular Consultants Ltd., Minneapolis, MN. Department of Medicine, University of Minnesota, MN
5. Hennipen County Medical Center and Dept. of Medicine, University of Minnesota, MN
Abstract
In 1932, Bremer of Harvard filmed the blood in the very early embryo circulating in self-propelled mode in spiralling streams before the heart was functioning. Amazingly, he was so impressed with the spiralling nature of the blood flow pattern that he failed to realize that the phenomena before him had demolished the pressure propulsion principle. Earlier in 1920, Steiner, of the Goetheanum in Switzerland had pointed out in lectures to medical doctors that the heart was not a pump forcing inert blood to move with pressure but that the blood was propelled with its own biological momentum, as can be seen in the embryo, and boosts itself with “induced” momenta from the heart. He also stated that the pressure does not cause the blood to circulate but is caused by interrupting the circulation. Experimental corroboration of Steiner’s concepts in the embryo and adult is herein presented.
Introduction
The fact that the heart by itself is incapable of sustaining the circulation of the blood was known to physicians of antiquity. They looked for auxiliary forces of blood movement in various types of etherisation and pneumatisation or ensoulement of the blood on its passage through the heart and lungs. With the dawn of modern science and over the past three hundred years, such concepts became untenable. The mechanistic concept of the heart as a hydraulic pump prevailed and became firmly established around the middle of the nineteenth century.
The heart, an organ weighing about three hundred grams, is supposed to pump some eight thousand liters of blood per day at rest and much more during activity, without fatigue. In terms of mechanical work this represents the lifting of approximately 100 pounds one mile high! In terms of capillary flow, the heart is performing an even more prodigious task of forcing the blood with a viscosity five times greater than that of water through millions of capillaries with diameters often smaller than the red blood cells themselves! Clearly, such claims go beyond reason and imagination. Due to the complexity of the variables involved, it has been impossible to calculate the true peripheral resistance even of a single organ, let alone of the entire peripheral circulation. Also, the concept of a centralized pressure source (the heart) generating excessive pressure at its source, so that sufficient pressure remains at the remote capillaries, is not an elegant one.
Our understanding and therapy of the key areas of cardiovascular pathophysiology, such as septic shock, hypertension and myocardial ischemia are far from complete. The impact of spending billions of dollars on cardiovascular research using an erroneous premise is enormous. In relation to this, the efforts to construct a satisfactory artificial heart have yet to bear fruit. Within the confines of contemporary biological and medical thinking, the propulsive force of the blood remains a mystery. If the heart really does not furnish the blood with the total motive force, where is the source of the auxiliary force and what is its nature? The answer to those questions will foster a new level of understanding of the phenomena of life in the biological sciences and enable physicians to rediscover the human being which, all too often, many feel they have lost.
Overview
Implicit in the notion of pressure propulsion in the cardiovascular system are the following four major concepts.
(1) Blood is naturally inert and therefore must be forced to circulate.
(2) There is a random mix of the formed particles in the blood.
(3) The cells in the blood are under pressure at all times.
(4) The blood is amorphous and is forced to fill its vessels and thereby takes on their form.
However, there are observations that challenge these notions. It is seen that the blood has its own form, the vortex, which determines rather than conforms to the shape of the vascular lumen and circulates in the embryo with its own inherent biological momentum before the heart begins to function. Just as an inert vortex in nature pulses radially and longitudinally, we tentatively assume that blood is also free to pulse and is not subject to the pulse-restricting pressure implied in the pressure propulsion concept. The blood is not propelled by pressure but by its own biological momenta boosted by the heart.
When the heart begins to function, it enhances the blood’s momentum with spiraling impulses. The arteries serve a subsidiary mimical heart function by providing spiraling boosts to the circulating blood. In so doing the arteries dilate to receive the incoming blood and contract to deliver an impulse to increase the blood’s momentum.
History
The history of the pressure propulsion premise goes back to Galileo and Leonardo da Vinci. The concept of the heart functioning as a pressure pump that forces the blood, assumed to be amorphous and inanimate, into its vessels and taking on the shape of its vessels was suggested by Borelli 1, a student and a close friend of Galileo, who observed the spiraling heart and compared its function to wringing the water out of a wet cloth. Borelli did not confirm his conjecture with experiments but was supported by misleading drawings of the left ventricle found later in Leonardo’s work. In Leonardo’s Notebooks the left ventricle wall was shown to be of uniform thickness as one expects to find in a pressure chamber.
However, quite the contrary, the left ventricle wall thickness varies by about 1800%, as we found by dissecting bovine hearts. The thickness ranges from 0.23 cm in the apex to 4.3 cm in the equatorial area. The apex wall is so soft and weak that it can be pierced with the index finger. The peculiar variability in the ventricular wall thickness is not in keeping with the idea of the heart being a pressure generator. However, one could conceive of such a wall configuration as maximizing the moment inertia with no static pressure in the ventricle.The thin, flexible, cone shaped apex and suspension from the aorta suggest the accommodation of a twisting function especially, when taking into account the spiral orientation of the myocardial muscle layers2.
The rotary motion of the heart, arteries, and blood has been measured or detected by several investigators 2, 18, 19. With slight variations, the erroneous sketch in Leonardo’s Notebooks has been used in most biology, physiology, and medical texts during the last few hundred years as well as in most modern anatomy texts in the last decades. Thus, false sketches have served to bear witness to a false premise.
William Harvey (1578-1657) attended the University of Padua while Galileo was on its Faculty. He seemed to be deciding in favor of momentum propulsion from his own experiments focusing on the blood flow and pressure propulsion probably under the influence of Borelli who focused on heart motion. At times he implied a momentum propulsion concept: “The auricle (atria) throws the blood into the ventricle” and “the ventricle projects the moving blood into the aorta.” “The blood is projected by each pulsation of the heart.” At other times he used expressions that imply a pressure propulsion concept. “The heart squeezes out the blood.” “The blood is forced into the aorta by contraction of the ventricle.” In a few cases he speaks of the pressure of the blood. However, he also used neutral terms, “the blood is transferred, transfused, transmitted, and sent” – from place to place.
Subsequent investigators who helped to firmly establish the pressure propulsion concept were as follows: Stephen Hales (1677-1761) who inserted a glass tube into the artery of a horse and assumed that the column of blood was balanced out by static pressure. Jean-Leonard-Marie Poiseuille (1799-1869) discovered that arterial dilation was in phase with ventricular ejection. Therefore, he assumed that the dilation was the passive response to the pressure in the blood. Among other things he substituted a mercury manometer for the blood manometer of Hales. Carl Ludwig (1816-1895) invented the recording manometer by adding a float with writing pen and moving chart to Poiseuille’s mercury manometer, and ushered in the age of continuous pressure recording. Finally, Scipione Riva-Rocci (1896-1903) perfected the sphygmomanometer in 1903 and brought the consideration of blood pressure into clinical practice.
The Problem and Its Proposed Solution
The problematic situation in cardiovascular physiology was expressed by Berne and Levy 3 who wrote: “The problem of treating pulsatile flow through the cardiovascular system in precise mathematical terms is virtually insuperable.” A fundamental aspect of this problem relates to the fact that the major portion of our knowledge of cardiac dynamics has been deduced from pressure curves. In fact our knowledge of the system has two independent sources: experimentally determined facts and logically deduced concepts from the pressure propulsion premise. The situation is so confusing that some life scientists are considering chaos theory and mathematics to try to find the order in the system. It will be shown that the chaos derives from a mix of facts and conjectures and not from the nature of the phenomenon itself.
It is our purpose to demonstrate that Borelli’s premise is incorrect and to propose the concept that the blood is propelled by a unique form of momentum. First, the aortic arch does not respond as expected if the blood in it were under pressure. The aorta is a curved tube; as such it has the basic form of the widely used pressure sensitive element of the Bourdon tube gage*.
When the curved tube of the Bourdon gage is subject to positive pressure, it is forced to straighten out as one sees in a garden hose. When subject to a negative pressure, the tube’s curvature is increased. During the systolic ejection (period when blood is ejected from ventricle), the aorta’s curvature is seen to increase, signifying that the aorta is not undergoing a positive pressure, but rather is undergoing a negative pressure 4.
We demonstrate that this negative pressure is that associated with the vacuum center of traveling vortices of blood. Thus the motion of the aorta, when considered as nature’s own pressure sensor, contradicts the pressure propulsion premise. Of course, the swirling streams of the vortex have potential pressure, so any attempt to measure pressure will result in a positive pressure reading due to interrupted momenta.
Movement without applied pressure is movement with momentum, as we observe so dramatically in the long leaps of racing cats. It is also manifest in nature in flowing water in open streams, traveling tornadoes, and jet streams which are actually horizontal spirals of air and moisture that can be thousands of miles long and move around like meandering rivers in the upper atmosphere. A thrown ball in its trajectory also moves without pressure.
What about the measured blood pressure? The concept under consideration here is the well known ratio of force to area:
pressure = force/area (force per unit area)
The pressure is an arithmetical ratio derived from the average force of the moving blood, and as such, indicates the phenomenon of the moving blood indirectly. In a momentum system the pressure is a potential while the object is in motion and becomes manifest when the velocity is impeded:
momentum (mass x velocity) = impulse (force x time)
The blood moves with various velocities in its vortex streams. At the moment of impact of an object moving with momentum, the velocity decreases while the pressure of a certain magnitude appears.
Rudolf Steiner, scientist and philosopher, pointed out on several occasions that the blood moves autonomously 5, and that the pressure is not the cause of blood flow but the result of it 6. The clinicians of old used elaborate methods of describing the nature of the arterial pulse and the ictus cordis or the apex beat, which is the impulse of the heart against the chest wall. Many descriptive terms such as thready pulse of hypovolemic shock, collapsing or water-hammer pulse of aortic incompetence and heaving apical impulse of left ventricular hypertrophy, convey the intuitive understanding of the real mechanism of the heart’s action.
An attempt to characterize left ventricular function by indices such as the maximal velocity of contraction (Vmax) and the maximum change of left ventricular pressure with time (dP/dtmax) suggests the felt inadequacy of the simple pressure propulsion concept.
Flow and Pressure Considerations
When fluid mass is subject to force in the form of a pressure, it will first resist movement because of its inertia and viscosity. In a pressure driven system the pressure rises faster than the fluid moves; the pressure will peak before the fluid velocity peaks. However, when one simultaneously measures pressure and flow in the aorta, the peak flow markedly precedes the peak pressure. This phenomenon was observed as early as 1860 by Chauveau and Lortet and, as reported by McDonald 7, it contradicts the law of inertia in the pressure propulsion concept. (See Fig. 2.) While this phase relationship actually confirms the momentum propulsion principle, it nevertheless remained a source of conjecture for a considerable period of time in the 1950s until it was rescued with the help of elaborate mathematical modeling for oscillating flow.
An observation in favor of the concept of the blood having its own momentum was reported by Noble 8 in 1968. By simultaneous pressure measurements in the left ventricle and the root of the aorta of a dog, he demonstrated that the pressure in the left ventricle exceeds the aortic pressure only during the first half of the systole and that the aortic pressure is actually higher during the second half. He found it paradoxical that the ejected blood from the ventricle continues into the aorta despite the positive pressure gradient. The erroneous concept of left ventricular pressure exceeding the aortic pressure during entire systole proposed by Wiggers in 1928 is still depicted in many modern texts of physiology. Noble proposed that this type of pressure pattern could be a result of momentum flow; however, this idea was overshadowed by the edifice of pressure propulsion.
The concept of pressure propulsion sent physiologists and scientists from diverse fields on a crusade that resulted in numerous hypotheses and theories about the cardiovascular system mechanics. The saying that, fluid dynamists in the nineteenth century were divided into hydraulic engineers who observed what could not be explained and mathematicians who explained things that could not be observed, still stands true to this very day.
Embryological Observations
Steiner 6 indicated that embryology provides the clues for solving the problem of the circulation. In relation to this, Bremer 9performed a remarkable series of observations of blood circulation in the very early chick embryo before the formation of the heart valves. He described the two streams of spiraling blood with different forward velocities in the single tube stage heart. Nevertheless, the blood is noted to have a definite direction of flow within the conduits and moves without an apparent propelling mechanism.These streams spiral around their own longitudinal axes and around each other. The streams appear to be a considerable distance apart, do not fill their vessels, and appear to be in discontinuous segments.
In a movie made by Bremer of the beating embryonic heart, one observes that the spiraling blood is boosted by the pulsating heart without creating turbulence in the blood. This suggests that the momentum transfer occurring between the heart and blood is in phase; the heart must somehow sense the motion of the blood and respond to it in turn with a spiraling impulses at the same velocities as the blood, thereby combining blood and heart momenta.
It is assumed that heart muscle layers have the same velocity distribution pattern as the concentric streams of a free vortex to enable heart and blood motions to couple in multi-velocity phase. It was significant to observe that the movement of the heart occurred with minimal inward motion of the heart wall. That the streaming of the blood can be observed before the functioning of the heart is supported by observations that the circulation in the early chick embryo is maintained for around 10 minutes after the heart had been excised 10. Moreover, the inherent mobility of the blood was highlighted by Pomerance and Davies 11, who found an embryo that lived to term without a heart but was born dead and grossly disfigured. Thus, the composite view of the embryonic cardiovascular system tells us that the blood is not propelled by pressure, but rather moves with its own biological momentum and with its own intrinsic flow pattern.
Alternations of Liquid and Gas Vortices in the Blood
The existence of apparently empty space between and within the spiraling liquid stream can be explained as space filled with gas or vapor. However, this hypothesis appears absurd when considering that even small bubbles in the arterial side of circulation can result in significant embolism. Each 100 cm of arterial blood contains 0.3 ml of free physically dissolved oxygen, 2.6 ml of carbon dioxide and 1 ml of nitrogen.
The importance of the small amount of dissolved oxygen is recognized only in extreme cases of anemia when it becomes a significant alternative source of tissue oxygenation. When viewed in terms of a highly differentiated distribution of solid, liquid and vapor/gas components of the composite vortex, this amount of free gas assumes critical importance.
The fact that the gas is elusive in the escaping liquid blood is very much in accord with the finding that the blood, as individualized liquid and gas vortices, moves with pressure-free momentum. The vortex in tornadoes is a very stable cohesive configuration with a vacuum center strongly held together by a centripetal force system. It does not have the physical properties of amorphous gas under pressure that tends to expand.
To further elucidate our observations, we contrived a model ventricle with a sealed, inverted cone-shaped, 0.5 liter clear glass flask filled with water. The instrumentation consisted of installing two tubes within the flask connected to pressure transducers to record vacuum in the vortex center and the potential pressure impulse in the momentum of the swirling water. The signal of pressure versus time was displayed on the oscilloscope screen and also fed to the computer for further analysis. The ventricle was operated by holding it in the hand and giving it a wobble and twist simultaneously to create a vortex. To enhance visibility, we filled the canister with methylene blue colored water.
Even the most energetic operation resulted in virtually no motion of the water. With some experimenting we determined that unless the model ventricle had about 1/3 of its volume as air space, a vortex could not be formed. This led us to reason that the highly organized gas/rarified plasma is a necessary component of the blood vortex. This also raises the question of how the gas and fluid elements can express the life property of locomotion.
The idea of the composite blood cells-plasma-gas vortex is in accord with the gaps in the flow of the embryonic vessels. To evaluate how valid our model ventricle was, we measured its potential impulse pressure (blood pressure as it is typically measured) in the swirling water and the vacuum in its center and found them to be in the range of +130 to -180 mm Hg, respectively.
Furthermore, we constructed a glass `ventricle’ with an attached `aorta’ and showed that up to 50% of the volume of the liquid could be ejected by subjecting it to a rotary-wobbling impulse, without the inward motion of the `ventricular’ wall.
A Well Known Vortex Function
It is well known that the pattern of blood flow through the heart significantly contributes to heart valve dynamics as has been shown by several studies utilizing contrast cineradiography and more recently color Doppler imaging. Taylor and Wade 12 confirmed stable vortex flow patterns behind the cusps of mitral and tricuspid valves visualizing the fine stream contrast injection. Furthermore, the vortex formation in the aortic sinus has not only been demonstrated in the model heart, but also visualized with three-directional magnetic resonance velocity mapping 13. Without the vortex formation in the aortic sinus, it is conceivable that with the blood rushing out of the left ventricular outflow tract at one to two meters per second, the coronary arteries would be ill perfused, as is the case in severe aortic stenosis (narrowing), where high velocity blood flow does not allow for formation of the normal supravalvular vortices.
Evidence of Momentum Flow in the Adult
Not only is the blood flow well maintained in the embryo before the formation of the valves; there are reports of adults in whom both infected tricuspid and pulmonary valves were surgically removed and not replaced by prosthetic valves, without significant problems 14. Werner et al. 15 using two dimensional echocardiography observed that the mitral and aortic valves were open during external chest compression and that cardiac chambers were passive and did not change in size.
The Perpetual Vortex in the Ventricle
The widely used technique of cardiac output measurement using the thermodilution method is fraught with significant deviations of individual measurements. This technique is based on the principle of warm blood mixing with the bolus of cold saline in the ventricle and detecting the rise in temperature of the resulting mixture in the pulmonary artery. A final value is obtained by averaging the results of several measurements.
By measuring electrical conductivity at various locations in the left ventricle of a dog, Irisawa 16 was unable to show uniform mixing of saline. The conductivity records showed the swirling streams of blood of different concentrations of saline within the ventricles during systole and diastole (the dilation or expansion stage of the heart muscles that allows the heart cavities to fill with blood), further supporting the concept of the highly organized vortical patterns inside the chambers of the heart.
Brecher 17 conducted an experiment on a dog that demonstrated a region of continuous negative pressure in the ventricle by observing the continuous flow of Ringer’s solution from a vessel outside the heart through a cannula positioned in the left ventricle via the atrial auricle. This further confirms our concept of the persistence of the vortex in the ventricle with its negative pressure center and positive pressure impulse potential in its swirling periphery throughout the cardiac cycle. Thus the heart as a minimum functional organ consists not only of its tissue but also of the perpetual vortex of blood which provides the perpetual vacuum in its center that probably helps to pull the blood back to the heart from capillaries and veins. The persistence of the vortex explains the anomaly to engineers of a supposed pump that retains 40 % of its charge with each ejection; a pump is expected to eject close to 100 % of its charge. As a pump concept it is absurd; as presented herein it is ingenious. Pettigrew 2 found three columns of spiraling blood in the left ventricle.
Orbiting Blood Corpuscles
In contrast to the parabolic velocity profile assumed by small particle suspensions in rigid tubes of small diameter under pressure, the cellular elements in the blood arrange themselves in a flow pattern in vivo, such that the heavier red blood cells orbit nearest the center with lighter platelets in more distant orbits surrounded by a sleeve of plasma at the vessel wall. Such an ordered arrangement of blood particle configuration in a sectional view of the arteries denies an omnidirectional pressure propulsion mechanism and confirms the vortex/momenta premise.
One can demonstrate this phenomenon of differentiation by mass in the vortex by allowing spheres chosen for convenience, same size (3 mm diameter), differently colored for different weight, to swirl freely in water. It will be seen that the heaviest spheres orbit nearest the center of rotation. The vortex orbital velocities increase as the orbits approach the center of rotation. On the contrary, during the time that a force couple is applied to rotate the vessel, creating a forced vortex, all of the spheres are forced out to the periphery where the velocities are the greatest as in a centrifuge.
To further confirm the existence of the free vortex velocity pattern in vivo, we probed the blood flow in the carotid artery by positioning a Doppler transducer at 900 to the wall to sense the blood’s swirling motion and processed the Doppler echoes through a variable band pass filter looking for frequency (velocity) distribution patterns. We detected echoes from groupings of particles at 400 to 650 Hz, 650 to 900 Hz and below 200 Hz Doppler-shifted frequencies. These three groupings indicate three separate orbital regions and velocities. Preliminary observations point to a highly ordered distribution of the blood’s cellular and plasma components.
Also, when moving through larger arteries the red cells are in toroidal shape, with their mass at the periphery to maximize the moment of inertia, and are assumed to rotate about their individual axes due to the phenomenon of vorticity (the creation of micro-vortices between swirling layers in the main vortex moving at different velocities). Thus we can expect to find that the billions of red cells are actually traveling in their own unique space as further evidence of the extreme order of the blood motion.
The Spiral Theme
The spiral theme is also apparent in the heart and vessel form and function. The musculature of the heart and arteries all the way down to the pre-capillaries is spirally oriented, and both the heart and arteries move spirally to augment the momenta of the blood 2,(18), 19. The literature on anatomical and physiological considerations of the twisting motion of the heart and vessels is comprehensive and has recently been reviewed 2. The fact that arterial endothelial cell orientation closely follows the blood flow patterns is well established 18, (19).
In a group of patients undergoing reconstructive vascular surgery of the lower extremities, Stonebridge and Brophy observed by direct angioscopic examination that the inner surface of arteries was organized in a series of spiral folds that sometimes protruded into the lumina. They commented that the folds occur as a result of spiral blood flow, which may be more efficient, requiring less energy to drive the blood through tapering and branching arterial system 19. They also observed the vortexing blood with fiber optics in the region of the endoluminal folds. In relation to this, enthusiasts know that rifled gun barrels forcing spin on the bullet make it more stable in flight and therefore more accurate in reaching its target. In the vessels the blood grooves its own conduits for the purpose of enhancing its torsional impulse. However, these spiral folds are not found in excised arteries; they are dynamics of living tissue.
Physiological Conclusions
The autonomic vortex movement of the blood discussed herein is inherent to the blood motion. It is not an accidental local disturbance often explained as turbulence or eddy currents, nor a localized phenomena with a single functional purpose as in heart valve dynamics. From a broader view it is to be expected that blood should so move, considering that fluids in nature tend to move curvilinearly, which is their path of least energy. The extreme expression of this tendency in nature, in terms of order, stability and minimal expenditure of energy are tornados and jet streams.
Potential Clinical Consequences
These observations should foster an accelerated understanding of the cardiovascular system through a reexamination of the vast amount of valuable experimental data gathered world wide. Since we have observed that the blood has a highly ordered dynamic form and an ordered blood corpuscle motion, and orientation, we should be able to develop devices and techniques to detect small deviations from group and individual norms and thus form a basis for very early diagnosis of cardiovascular disease, which remains the number one cause of death in the U.S. Novel, more effective therapies for cardiovascular disease hopefully will also evolve from this new perspective on cardiovascular physiology.
End notes
* The Bourdon tube gage is named after its inventor, Bourdon. Its pressure sensitive element consists of a circularly bent tube that is flattened to increase its sensitivity to pressure. When the tube is subjected to an internal positive pressure it tends to straighten; when subjected to an internal negative pressure its radius of curvature is increased. The deformation of the tube is proportional to the pressure and is transmitted via links and gears to motions that turn a pointer on a scale calibrated to indicate pressure.
Acknowledgments
We thank Larry W. Stephenson, M.D., Chief of Cardiothoracic Surgery, Wayne State University School of Medicine, and Beverly Rubik, Ph.D., for their comments on this work.
References
1. Borelli, De Motu Animalium. Rome, 1681.
2. Marinelli, R., Penney, D.G., et al. 1991. Rotary motion in the heart and blood vessels: a review. Journal of Applied Cardiology 6: 421-431.
3. Berne, R., Levy, M., 1986. Cardiovascular Physiology. St. Louis, MO: C.V. Mossy Co., p. 105.
4. Rushmer, R.F., D.K. Crystal. 1951. Changes in configuration of the ventricular chambers during cardiac cycle. Circulation 4: 211-218.
5. Steiner, R., 1990. Psychoanalysis and Spiritual Psychology. Hudson, NY: Anthroposophic Press, p. 126.
6. Steiner, R., 1920. Spiritual Science and Medicine. London, England: Rudolf Steiner Press, 24-25.
7. McDonald, D.,1952. The velocity of blood flow in the rabbit aorta studied with high speed cinematography. Journal of Physiology 118: 328-329.
8. Noble, M.I., 1968. The contribution of blood momentum to left ventricular ejection in dog. Circulation Res. 26: 663-670.
9. Bremer, J. 1932. Presence and influence of spiral streams in the heart of the chick embryo. American Journal of Anatomy, 49: 409-440.
10. Manteuffel-Szoege, L., 1969. Remarks on blood flow. J. of Cardiovasc. Surg. 10: 22-30.
11. Pomerance, A., Davies, M. 1975. Pathology of the Heart London, England: Blackwell Scientific Publications, pp. 538-39.
12. Taylor, D.E.M., J.D. Wade. 1973. Pattern of blood flow in the heart. Cardiovascular Research 7:14-21.
13. Kilner P.J., Z. Y. Guang, et al. 1993. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic velocity mapping. Circulation 88: 2235-2247.
14. Arbulu, A., I. Asfaw. 1981. Tricuspid valvulectomy without prosthetic replacement. J. Thorac Cardiovasc Surg 82: 684-691.
15. Werner, J.A., H.L. Greene, et al. 1981. Visualization of cardiac valve motion in man during external chest compression using two dimensional echocardiography. Circulation 63: 1417-1421.
16. Irisawa, H., M. F., Wilson, R.F. Rushmer. 1960. Left ventricle as mixing chamber. Circulation Research 8:183-87.
17. Brecher,G.A. 1956. Experimental evidence of ventricular diastolic suction. Circulation Research 4:513-18.
18. Lowell, L.B., L.S. Adamson. 1980. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ. Res. 48: 481-488.
19. Stonebridge, P.A., C. M. Brophy. 1991. Spiral flow in arteries? The Lancet 338:1360-61.